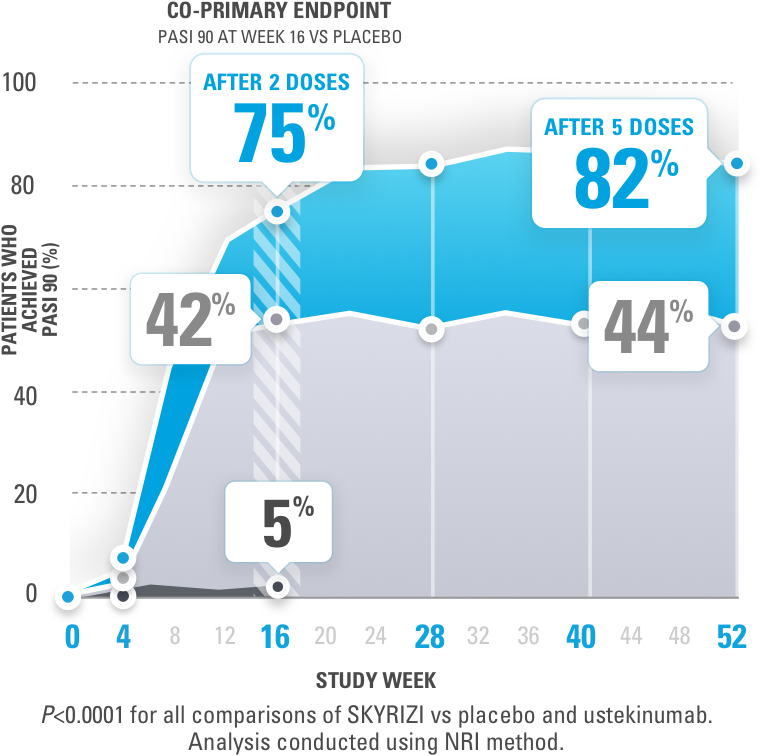
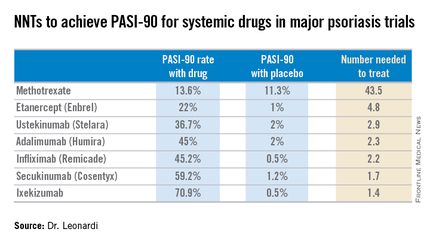
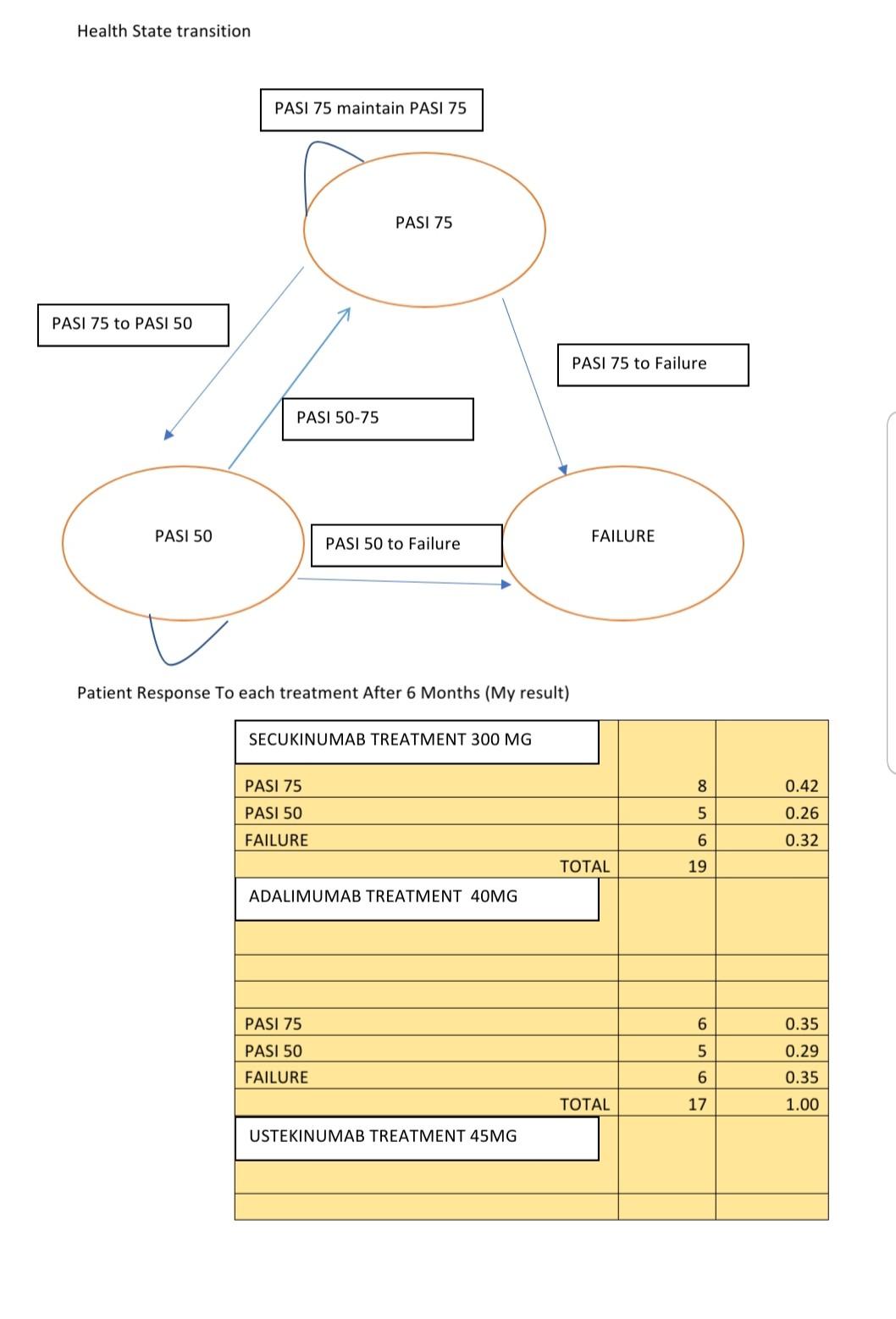
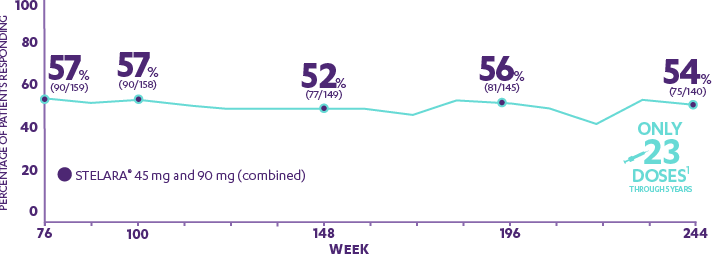
Adalimumab for long-term treatment of psoriatic arthritis: 2-year data from the Adalimumab Effectiveness in Psoriatic Arthritis Trial (ADEPT) | Annals of the Rheumatic Diseases

Ixekizumab sustains high level of efficacy and favourable safety profile over 4 years in patients with moderate psoriasis: results from UNCOVER‐3 study - Lebwohl - 2020 - Journal of the European Academy
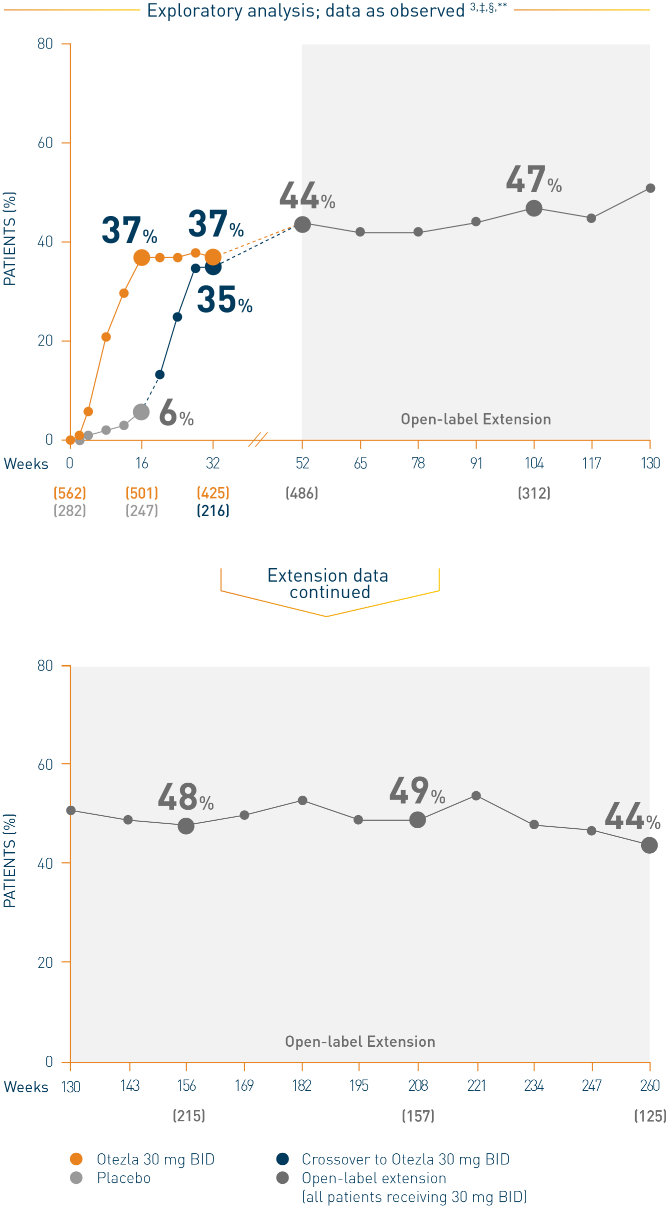
PASI-75 Response with Otezla in the Treatment of Moderate to Severe Plaque Psoriasis — Efficacy | Otezla® (apremilast) Healthcare Professional Site

Health-Related Quality of Life Worsens Disproportionately to Objective Signs of Psoriasis After Withdrawal of Adalimumab Therapy | SpringerLink

Long-term efficacy and safety of ixekizumab: A 5-year analysis of the UNCOVER-3 randomized controlled trial - ScienceDirect

Ixekizumab, an interleukin-17A specific monoclonal antibody, for the treatment of biologic-naive patients with active psoriatic arthritis: results from the 24-week randomised, double-blind, placebo-controlled and active (adalimumab)-controlled period ...
Real-world efficacy of biological agents in moderate-to-severe plaque psoriasis: An analysis of 75 patients in Taiwan | PLOS ONE

Improvement in Psoriasis Symptoms and Physical Functioning with Secukinumab Compared with Placebo and Etanercept in Subjects with Moderate-to-Severe Plaque Psoriasis and Psoriatic Arthritis: Results of a Subanalysis from the Phase 3 Fixture

PASI 75, 90 and 100 Response Rate. (a) PASI 75. *P = 0.002 week 3; P <... | Download Scientific Diagram