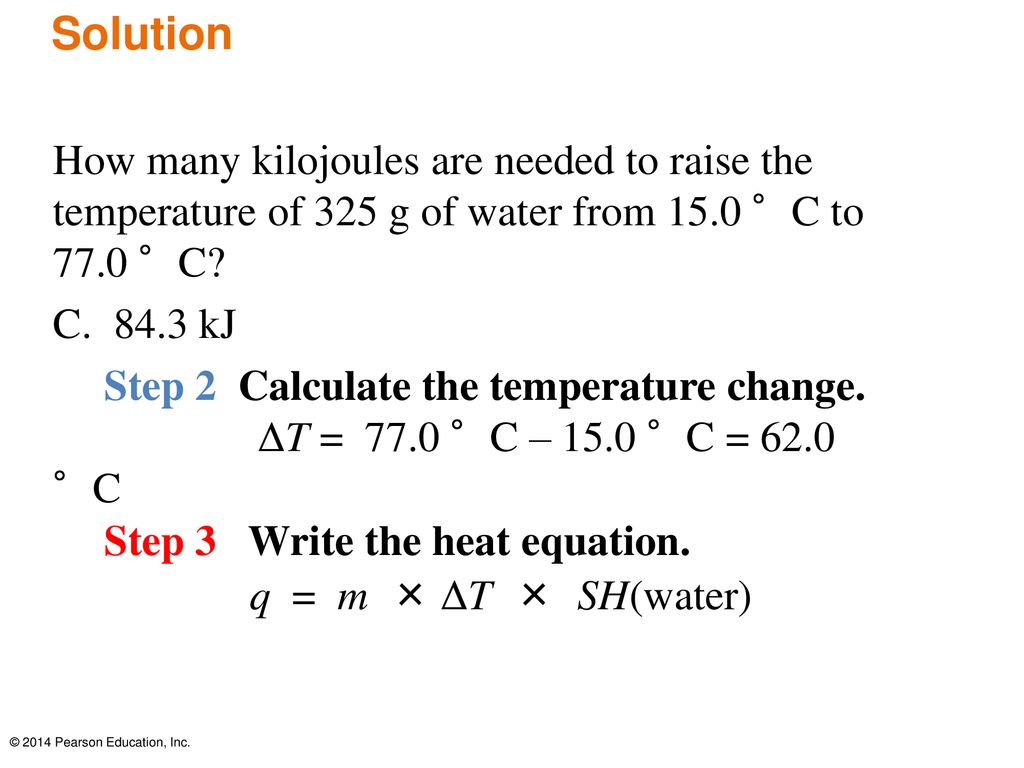
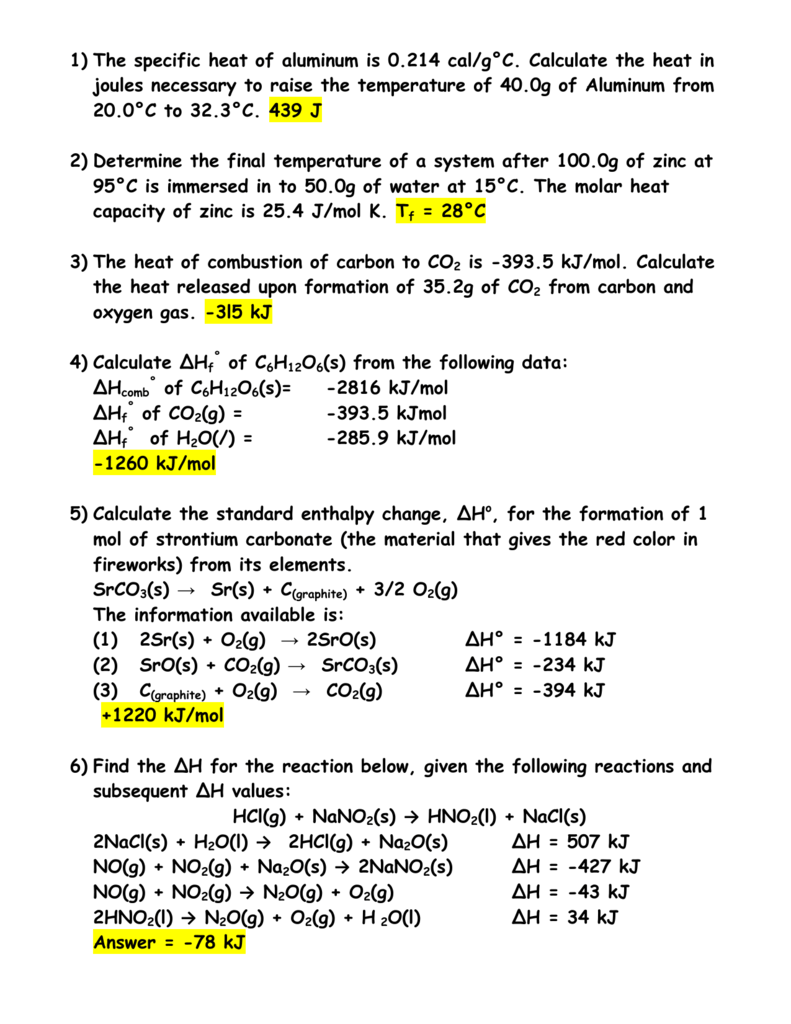
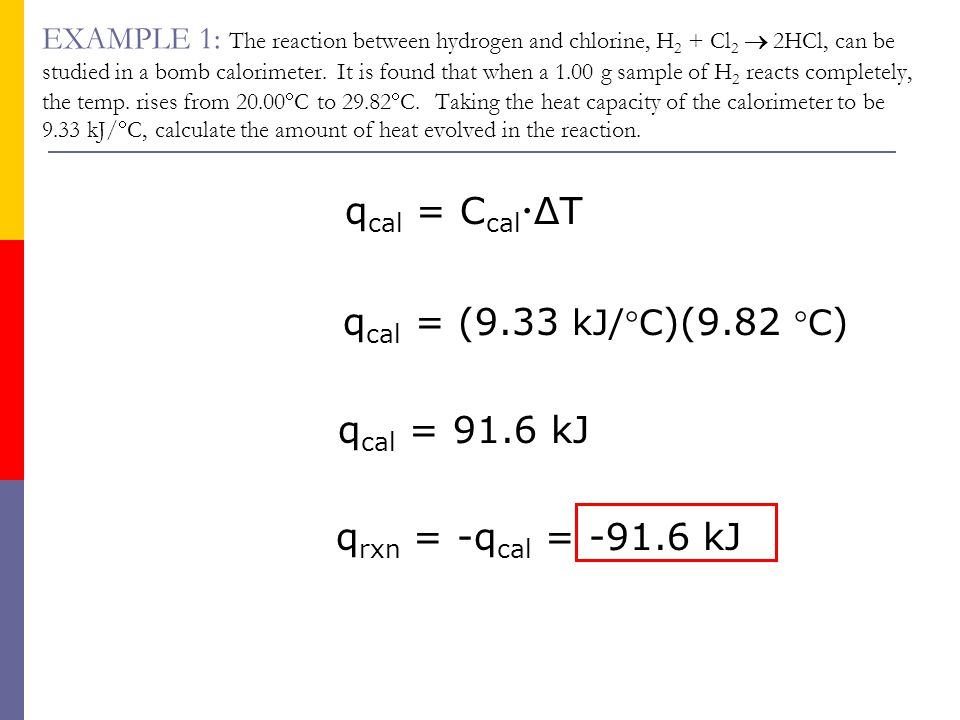
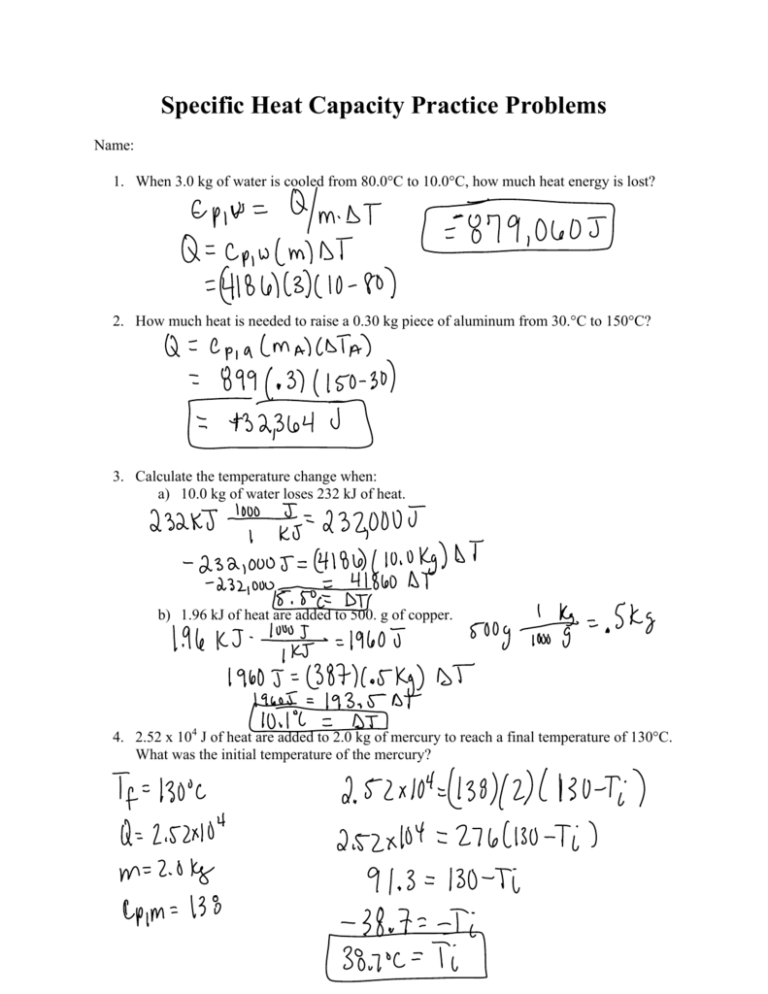
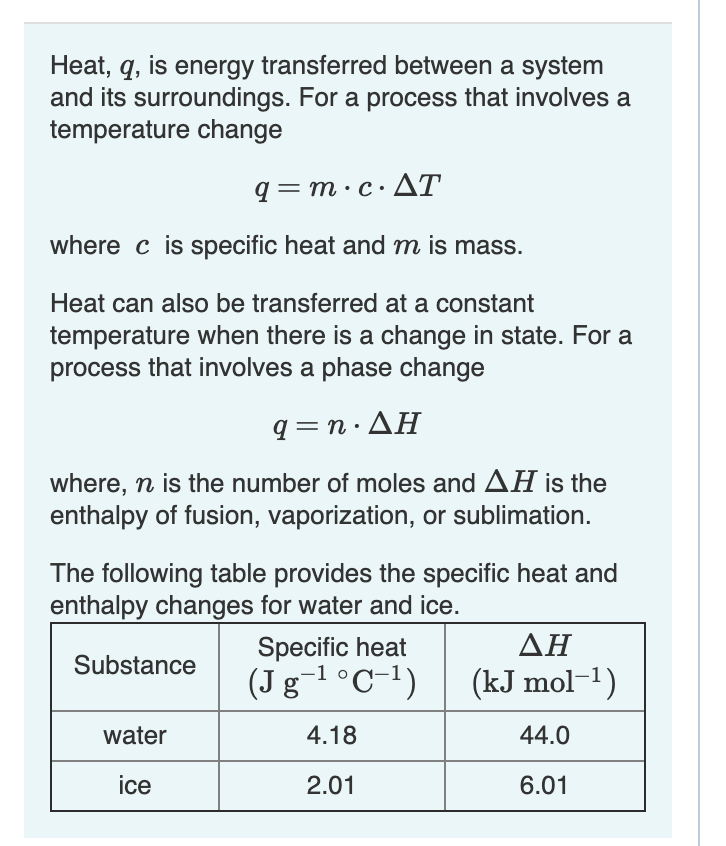
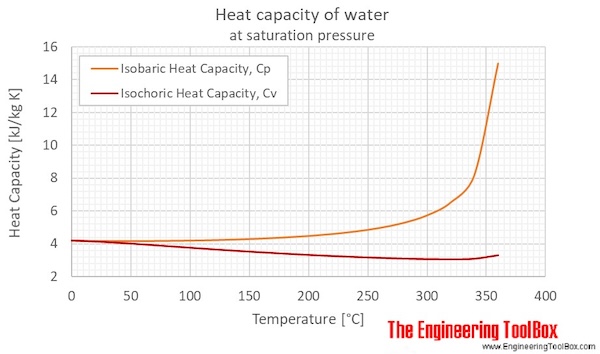
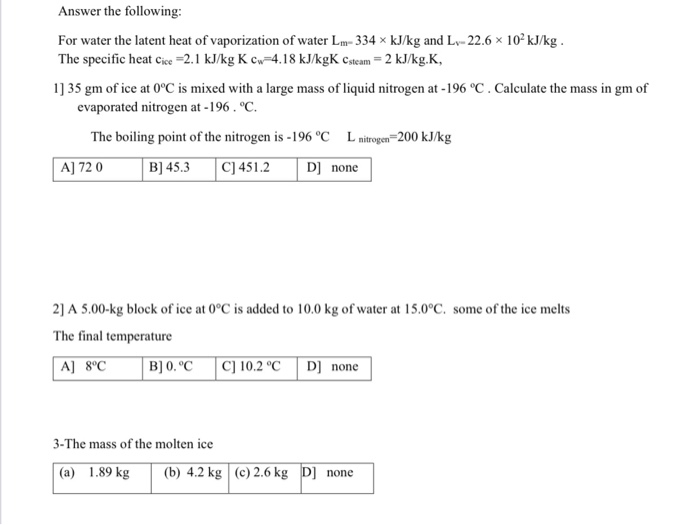
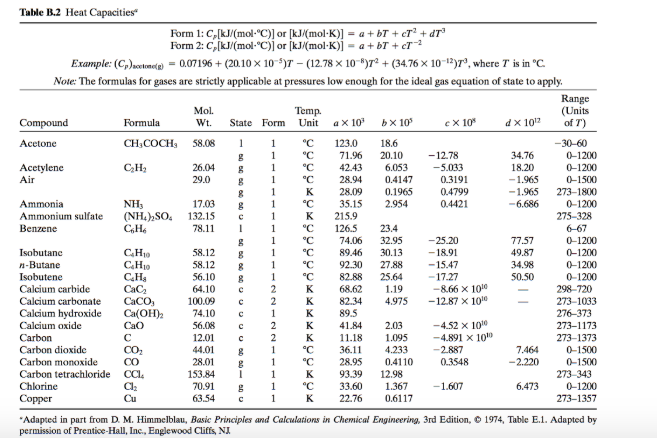
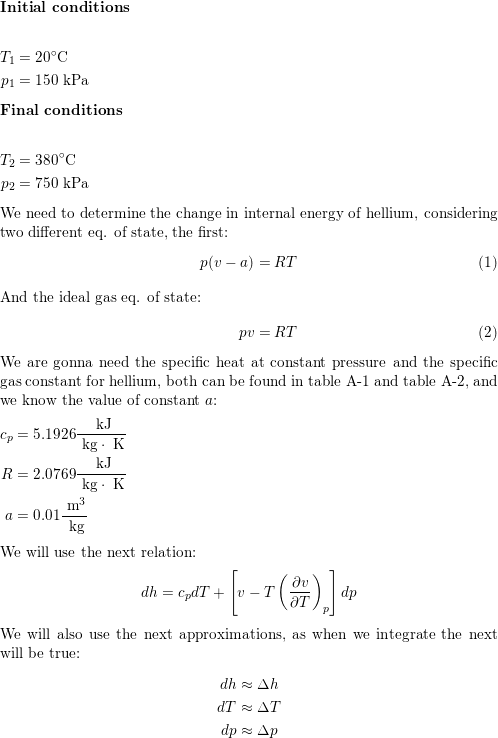26. For a reaction A+B - C. AH = 30 kJ mol and AS = 60 JK-mol-'. The temperature, above which reaction will be spontaneous is (1) 500°C (2) 227"C (3) 127°C (4) 175°C

Using the data provided, calculate the multiple bond energy (kJ mol^-1) of a C≡≡ C bond in C2H2 . Given that the heat of formation of C2H2 is 225 kJ mol^-1 (take
Solved] 1. An engine gains 550 KJ of heat from a hot source at 700 deg. celsius and rejected at 50 deg. celsius. What is the amount of the power out... | Course Hero
Solved] Determine the average Cp value in KJ/kg-K of a gas if 522 KJ/kg of heat is necessary to raise the temperature from 300K to 800K making the p... | Course Hero

Ridgid 62697 KJ-1750-C Water Jetter Drain Cleaner with Cart | RidgidToolsOnline | Ridgid Parts Online | Plumbing Tools | PlumbersCrib.com
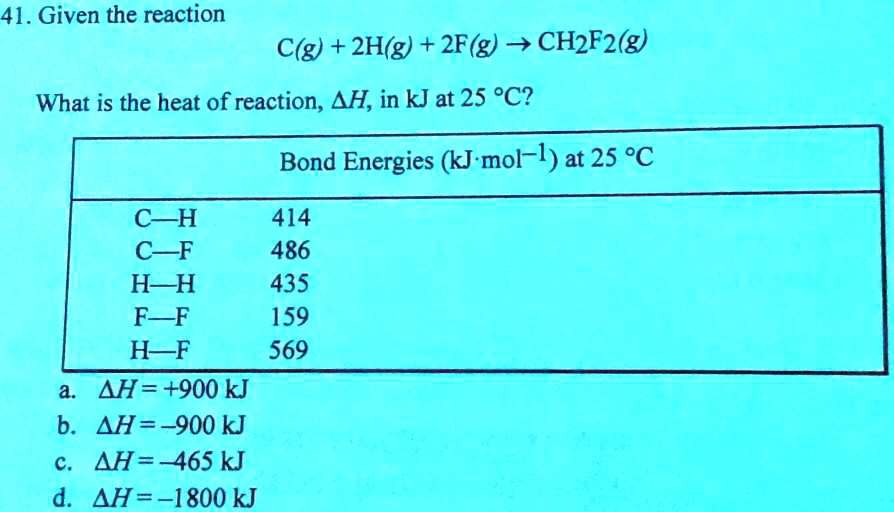
SOLVED:41. Given the reaction C(g) + 2H(g) + 2F(g) v CH2F2(g) What is the heat of reaction, AH, in kJ at 25 PC? Bond Energies (kJ mol-l) at 25 %C C-H C~F

Specific Heat Capacity. Specific Heat Capacity (kJ/kg/°C) The specific heat is the amount of heat (measured in kJ) per unit mass (measured in Kg) required. - ppt download

SOLVED:How much energy is needed to convert 59.5 grams of ice at 0.00 %C to water at 60.9 *C? Information: specific heat (ice) = 2.10 J/g "€ specific heat (water) = 4.18